AN REVIEW ‘DE RUSSAS’ ORANGE: CHARACTERISTICS, PROPAGATION AND PRODUCTION
Capítulo de livro publicado no livro: Ciência e tecnologia de alimentos: Pesquisas e avanços. Para acessa-lo clique aqui.
DOI: https://doi.org/10.53934/9786585062060-23
Este trabalho foi escrito por:
Sheyla Maria Barreto Amaral *; Felipe Sousa da Silva ; Marjorie Beatriz Vidal Maia ; Cleilson do Nascimento Uchôa ; Marlene Nunes Damaceno
Federal Institute of Education, Science and Technology of Ceará, Campus Limoeiro do Norte, Ceará, Brazil
*Corresponding author – Email: [email protected]
Abstract: There is a sweet orange variety in the state of Ceará in Brazil that is adapted to the semi-arid region called the ‘de Russas’ orange, cultivated in the Jaguaribe Valley region. Considered sweeter than the ‘Pera’ orange, this variety has been gaining space at consumers’ tables and has the potential to meet the state’s table fruit demand; however, it is only marketed in fresh form, without using processes which add value to the product, and there are no studies which detail its food characteristics. This study addresses an integrative literature review which highlights the sweet orange regarding its origin and botanical aspects; the characteristics and benefits of fruit and juice, which is the main derivative; production, export and consumption; and characteristics, propagation and production of the ‘de Russas’ orange variety. Its main objective is to convey information to academia and industry, contributing to diffuse cultural knowledge, the local sustainability and the marketing supply of this derivative. Based on this review, it is found that the ‘de Russas’ orange currently represents a significant example of family farming in the interior of the state of Ceará. In addition, that organic cultivation is another way of preserving family farming in the semi-arid region in Ceará, reducing hunger and social inequality, and in turn contributing to expand citrus in the state and increase valorization of the traditional ‘de Russas’ sweet orange variety.
Keywords: family farming; Citrus sinensis (L.) Osbeck; organic cultivation.
INTRODUCTION
Carolus Linnaeus described the Citrus genus in 1753, and since then its taxonomy and the number of extant species have been studied. Citrus fruits belong to the Rutaceae family, most commonly found in tropical regions and considered the most widespread in the world. These fruits include oranges, grapefruit, tangerines, pomelos, limes and lemons. The main fruit of the group is the sweet orange (Citrus sinensis (L.) Osbeck), responsible for about 70% of production and total consumption (1, 2).
According to data from the 2022 Brazilian Horti&Fruti Yearbook, oranges are one of the plant species with the greatest supply in Brazil, making it the world’s largest producer of the fruit and the largest exporter of juice in the world. The orange ranks first of the 21 species of fruit produced in the country (3).
Despite the prominent position that some states have in Brazilian citrus production, the center with the highest production is São Paulo and the Triângulo/Southwest Mineiro, better known as the citrus belt, present in 350 municipalities (4). Data from the General Register of Employed and Unemployed People (Cadastro Geral de Empregados e Desempregados – Caged) released by the National Association of Citrus Juice Exporters (CitrusBR) report that the Brazilian citrus industry was responsible for 38,327 admissions in 2020, which represents 10.23% of the jobs generated in the state of São Paulo, and 6.33% of the Brazilian total, one of the main sectors for job generation in the country (5).
According to the Systematic Survey of Agricultural Production (Levantamento Sistemático da Produção Agrícola – LSPA) released by the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística – IBGE), the Northeast Region is the second largest orange producer in the country, contributing with 1,251,948 tons in the 2022 harvest, preceded by the Region Southeast, which holds more than 80% of production (6).
Several factors negatively influence citrus expansion growing in the Northeast, such as: hot and dry weather; difficulty in acquiring cultivars that adapt to biotic and abiotic stresses and seedlings with genetic and phytosanitary quality; absence of fertile soils; appropriate water for irrigation; technical assistance and rural extension; and farmers’ lack of access to rural credit (7, 8).
Despite these obstacles, Ceará State is the fourth largest producer of oranges in the Northeast Region (9), and has the potential to regenerate and expand the production chain given the absence or control of the main diseases and pests of economic importance and diseases which affect the citrus industry worldwide (10).
Ceará has an orange variety adapted to the semi-arid region, called the ‘de Russas’ orange, making Russas – a municipality located in the Jaguaribe Valley region – recognized as the “land of oranges” (11). Despite this, the orange harvested area between the years 1990 and 2020 in this municipality was reduced from 70 to 36 hectares. On the other hand, the neighboring municipality, Jaguaruana, located on the East Coast of the State, showed an increase of more than 280% in the harvested area, from 30 to 106 hectares in the same period, being considered a potential producer of the crop in Ceará. Other municipalities are also producers, such as Quixeré, Limoeiro do Norte and Tabuleiro do Norte, indicating that production is not limited to the municipality of Russas, and has been gaining space in the orchards of the state (12).
Embrapa (Empresa Brasileira de Pesquisa Agropecuária) Mandioca e Fruticultura in Cruz das Almas (BA) has been studying the ‘de Russas’ orange variety cultivated in the form of unripe plants in the Vale do Jaguaribe region since the mid-1930s (13). This orange is considered sweeter than the ‘Pera’ orange, has been gaining space on consumers’ tables, and has the potential to meet the market demand for table fruit in the state (14).
However, although there are already 8 cultivars (BRS ‘Russas 01’, ‘02’, ‘03’, ‘04’, ‘05’, ‘06’, ‘07’, ‘08’) identified, selected and registered at the Ministry of Agriculture, Livestock and Supply (Ministério da Agricultura, Pecuária e Abastecimento – MAPA), it is only sold in natura, without using processes that add value to the product, and there are no studies which detail its food characteristics (15).
This study addresses an integrative literature review which highlights the ‘de Russas’ sweet orange variety in terms of origin and botanical aspects; characteristics and benefits of fruit and juice (its main derivative); production, export and consumption; and characteristics, propagation and production. Its main objective is to transmit information to academia and industry, contributing to disseminate cultural knowledge, local sustainability and the market offer of this derivative.
DEVELOPMENT
1 ORIGIN OF THE SWEET ORANGE
According to researchers, citrus fruits originate in East Asia from the southern slopes of the Himalayas to southern China, Indochina, Thailand, Malaysia and Indonesia (16, 17).
Studies indicate that the orange originated from the crossing of the pomelo (Citrus maxima Burm.) with the tangerine (Citrus reticulata Blanco) and that it was taken from Asia to North Africa in the Middle Ages, eventually reaching the south of Europe. Finally, it reached the Americas around the year 1500 (18, 19).
Citrus fruits were introduced into Europe with citrons in the 4th century BC, followed by lemon trees (10th century AD), sweet orange trees (15th century AD) and tangerine trees (19th century AD). Christopher Columbus was responsible for taking them to America, more precisely to Haiti, in 1493. Citrus arrived in South Africa in 1654; and in Australia in 1788 (20, 21).
The first introductions of the sweet orange were lost after the fall of the Roman Empire. Then it was reintroduced by the Genoese through their trade routes in 1425. Four types of sweet oranges are recognized: common, navel, pigmented and low-acid oranges (22).
Citrus fruits were introduced in Brazil around 1500 by the first Portuguese colonizing expeditions on the coast of Bahia. Citrus trees in Rio Grande do Sul were brought by the Azoreans in the Rio Taquari Valley in 1760, with seedlings of ungrafted trees, then expanding soon after to the Rio Caí Valley (23).
In a study on oranges in Brazil by Hasse (24) which considered the period from 1500 to 1987, the ‘Pera’ orange is only mentioned at the end of the 19th century with its origin in Rio de Janeiro, where it was most cultivated. Until that date, the ‘Pera’ orange had not been mentioned in any publication.
2 BOTANICAL ASPECTS OF THE ORANGE
The orange is one of the most cultivated fruits in the world. It belongs to the Rutaceae family and is divided into two groups: the sweet orange and the sour orange. The sweet orange (Citrus sinensis (L.) Osbeck) is the most important citrus species, and comprises most of the varieties that are cultivated in the world. The ‘Pera’, ‘Valencia’, ‘Natal’ and ‘Folha Murcha’ varieties stand out in Brazil, intended for direct consumption and juice production (25).
The orange tree is a medium-sized plant reaching an average of 5 to 10 meters in height, with a dense crown and a rounded base. Its leaves are aromatic, green and shiny, the flowers are white and small. The fruit has a rounded shape, orange peel and epicarp rich in pectin. It has several vesicles (buds) of juice protected by a wax film called a peel (flavedo), which contains the substances responsible for the aroma and color of the fruit. There are segments composed of juice vesicles in its inner edible part, in addition to the solid part (membranes and albedo), which is a source of fibers and bioactive compounds, as well as seeds (26, 27, 28).
The climate exerts a direct influence on the quality and composition of the fruits, such as the amount of juice present, the size of the fruit, maturation and solids content. The orange adapts easily in Brazil given the growing conditions, soil and climate. The ideal temperature range for obtaining a fruit with the required quality ranges from 22 to 33 ºC (never above 36 ºC and never below 12 ºC) with an annual average of around 25 ºC (29).
Well-distributed rainfall throughout the year contributes to the crop quality, ideally with precipitation of around 1,200 mm occurring. If there is a water deficit, artificial irrigation can be used to correct it. It is preferable that the air humidity is around 60 to 80%. Also, one must think about the influence of the soil, although the orange tree manages to develop in different types of soils: sandy, clayey, deep and permeable, the plant adapts better in sandy-clay, deep and well-drained soils, and shallow soils with the possibility of waterlogging should be avoided (30, 31).
3 CHARACTERISTICS AND BENEFITS OF THE ORANGE AND JUICE
3.1 Nutritional composition
The most effective way to acquire the benefits that the orange has is to consume it in the form of juice or in natura. Some studies indicate that eating two oranges a day provides the full amount of vitamin C that the human body needs (19).
According to the Brazilian Food Composition Table (32), fresh ‘Pera’ orange juice (the most produced variety in Brazil) in a 100 g portion has a nutritional composition of 91.3 g.100 g-1 of water; 7.6 g.100 g-1 of carbohydrates; 0.7 g.100 g-1 of protein; 0.1 g.100 g-1 of lipids; 0.3 g.100 g-1 ash and trace amounts of dietary fiber. Regarding minerals and vitamins, 7 mg.100 g-1 of calcium; 14 mg.100 g-1 of phosphorus; 8 mg.100 g-1 of magnesium; 149 mg.100 g-1 of potassium; 0.03 mg.100 g-1 of manganese; 0.01 mg.100 g-1 of copper; trace amount of iron, sodium and zinc and B1, B2, B3 and B6 vitamins; 73.3 mg.100 g-1 of vitamin C; and a total caloric value of 33 kcal.
According to the Resolution of the Collegiate Board no. 269, of September 22, 2005, the Recommended Daily Intake (RDI) of vitamin C for adults is 45 mg (33), meaning that depending on the variety, growing conditions, post-harvest care, and the preparation method, only a portion of 100 g of orange juice provides the RDI for this nutrient.
In addition to the wide range of mentioned nutritional constituents, the orange also has a high content of micronutrients and phytochemicals, including substances with antioxidant properties (34). Several studies prove that the benefits of eating oranges and its juice come from vitamin C, phenolic compounds, flavonoids and carotenoids, which are bioactive compounds that act in strengthening the immune system (35, 36).
Furthermore, the benefits related to ꞵ-carotene, a bioactive compound that gives orange its color, are cited in the literature and can help prevent cancer and heart attacks (37). Consumption of juice can help reduce bad cholesterol (low density lipoprotein – LDL) and increase good cholesterol (high density lipoprotein – HDL) (38).
The antioxidant components of oranges improve blood vessel functioning, and fighting cardiovascular diseases (39). The fibers present in the skin, more precisely the pectin (white part), help with digestion (40). The calcium present in the orange pomace helps in maintaining bones, in forming muscles and blood, and the iron acts in forming hemoglobin and in oxygen transport (41).
Orange juice is the main derivative of the fruit, Decree no. 6.871 regulates Law no. 8.918 (standardization, classification, registration, inspection, production and supervision of beverages), and defines it in art. 18 as: unfermented and undiluted beverage, obtained from the edible part of the orange (Citrus sinensis), through an appropriate technological process (42).
In order to obtain a quality product meeting the standards and requirements of the market, Normative Instruction no. 37, of October 1, 2018, of the Ministry of Agriculture, Livestock and Supply (MAPA) provides the parameter analyzes of fruit juice and pulp and the listing of fruits and other complementary attributes to the already established identity and quality standards (43). The orange juice must meet the characteristics and composition described in Table 1.
Table 1 – Characteristics and composition of orange juice according to the Ministry of Agriculture, Livestock and Supply.
| Parameter | Minimum | Maximum |
| Soluble solids in °Brix, at 20° C | 10 | – |
| Ratio of soluble solids in °Brix/acidity in g.100 g-1 of anhydrous citric acid | 7 | – |
| Ascorbic acid (mg.100 mg-1) | 25 | – |
| Total natural orange sugars (g.100 g-1) | – | 13 |
| Orange essential oil (%v/v) | – | 0.035 |
Source: BRASIL (2018).
3.2 Quality attributes
The various characteristics of a fruit directly influence its quality, but its appearance gains prominence with regard to the commercialization of citrus for both in natura consumption and for processing (44). Fruit quality attributes relate to appearance, flavor, aroma, texture and nutritional value. The appearance is mainly associated with the external color variable, while the internal color also exerts influence for processing the fruit in elaborating derivatives (45).
The quality of a fruit is one of the main factors that determine its post-harvest destination. In the case of oranges, the following parameters are analyzed to verify their quality: chemical composition, including the content of vitamin C present, expressed in ascorbic acid, pH, soluble solids, titratable acidity, soluble solids/titratable acidity ratio (Ratio), color and fruit flavor (46).
These variables may change depending on the variety, soil, climate and post-harvest storage conditions, and must remain stable to ensure quality until it reaches the consumer’s table (47, 48). An alternative to control pre- and post-harvest factors is to monitor the levels of vitamin C present, helping to maintain the highest possible concentrations (49).
3.3 Ascorbic acid
Vitamin C or ascorbic acid is considered an agent with antioxidant potential, with the ability to inhibit the development of oxidative reactions caused in the human body (50, 34).
It is a water-soluble vitamin and is sensitive to high temperatures, rapidly degrading when exposed to oxygen, which is why it is considered an unstable antioxidant. Vitamin C undergoes degradation during food processing, which can be influenced by pH, exposure to light, oxygen and temperature in the face of chemical and biochemical reactions arising from processing and the final packaging and storage stages of the product (51, 52, 53).
Like other antioxidant compounds, the vitamin C content present in fruits is also influenced by environmental conditions, agricultural practices, plant genotype and fruit maturation index (54). Therefore, vitamin C is used as an indicator of quality and even conservation in fruits, vegetables and their derivatives, as it degrades more easily during storage than other compounds that may be present (55).
Orange juice plays an important role in achieving the Recommended Daily Intake of vitamin C, since human beings are not capable of synthesizing ascorbic acid in their bodies, requiring a dietary source to reach the RDI (56).
Several analysis techniques have been used to quantify the vitamin C present in fruit juices, such as titrimetry, fluorometry, spectrophotometry, and other more sensitive ones such as high-performance liquid chromatography (HPLC), which stands out due to its property of separating analytical interferents and speed in sample preparation, providing less conditions for the degradation of ascorbic acid (57, 58, 59).
3.4 Volatile organic compounds
Two other quality parameters which are also very important are the aroma and flavor of the product. These are sensory characteristics which please consumers, especially in the case of juice, as it is closely related to the aroma of fresh fruit, such as orange juice (60, 61, 62).
The constitution of fruit aroma comes from the release of low molecular weight (MW) volatile compounds which give off odors, which in turn are captured by the olfactory sensory system of human beings. The aroma is also associated with the parameters of color, texture and size of a fruit, and contributes to the consumer’s propensity to purchase it. It also enables differentiating varieties of the same species and characterizing their quality (63).
Volatile organic compounds (VOCs) have a hydrophobic character and low molecular mass, with approximately 20 carbon atoms, making them easily able to evaporate at room temperature, to cross cell membranes, and be released into the air or soil in the absence of a diffusion barrier (64).
VOCs belong to different chemical classes, mainly including compounds of terpene origin and derivatives of fatty acids, such as alcohols, ketones, lactones, aromatic compounds and esters, which form the food aroma in minimal combinations (65).
Terpenes make up a vast group of organic molecules, synthesized as secondary metabolites, mainly by plants, protecting them from attacks by external agents due to their antimicrobial action. They are chemically called hydrocarbons when composed of only carbon and hydrogen, but they are called terpenoids if they have oxygen in their structure, and perform various organic functions such as: acids, alcohols, aldehydes, ketones, ethers, phenols or terpene epoxides, and can still be cyclic or aromatic (66).
The terpenes are classified based on the amount of isoprene units in their structure (C5H8). Those with two isoprene units, or ten carbons, are called monoterpenes (absence of oxygen) or monoterpenoids (presence of oxygen) (67). Monoterpenes and monoterpenoids have prominent volatilization capacity which relevantly contribute to the aroma of natural products, especially citrus fruits, spices, condiments and aromatic herbs. However, terpenes with larger size molecules do not have such characteristics (68).
The volatile compounds were determined through gas chromatography coupled to mass spectrometry (GC-MS). Techniques emerged starting in the 1960s to evaluate the importance of each volatile compound in the aroma and taste of food (69, 70).
Figueiredo, Tocchini and Bordoni (71) were the pioneers in research with Brazilian juices, preparing concentrated orange juice and evaluating the volatile profile of the juices for 180 days, using the headspace-gas chromatography technique, and the sensory acceptance of the products. Brealey (72) and Mirhosseini et al. (73) quantified and qualified the volatile organic compounds present in oranges, among which α-Pinene, β-Pinene, 3-Carene, para-Cymene and D-Limonene stand out.
4 PRODUCTION, EXPORTATION AND CONSUMPTION
4.1 In natura orange
The production of citrus fruits is considered one of the most important commercial activities in many countries with tropical and subtropical climates such as Brazil, India and China, which are currently the main citrus producing countries in the world (34).
According to data from the Food and Agriculture Organization of the United Nations (FAO), Brazil leads the ranking of world production of oranges. Oranges remain in the 6th position of the ten best commodities in the country, with a production of 16,214,982 tons in 2021 (last update) (74).
Data from the Municipal Agricultural Production (Produção Agrícola Municipal – PAM) survey released by the Brazilian Institute of Geography and Statistics (IBGE) report that 574,563 hectares of orange trees were planted in Brazil in 2020, along with 572,698 hectares of harvested area. The country produced 16,707,897 tons of oranges, with an average yield of 29,174 kg per hectare, and a production value of R$10,898,251.00, accounting for 2.3% of the total production value of the main products of national agriculture, in turn occupying fourth position of the main products of the Southeast Region (75).
According to the report “Citrus: World Markets and Trade”, released by the Department of Agriculture of the United States (United States Department of Agriculture – USDA) in January 2022, a total of 8.0 million tons of fresh oranges were exported out of Brazil from July 2021 to January 2022. Brazil presented a consumption of 4.749 million tons of oranges, reporting that 30.259 million tons of oranges were consumed worldwide in the same period (76).
4.2 Frozen concentrated orange juice
Brazil remains the world’s largest producer and exporter of frozen concentrated orange juice (65 ºBrix) (frozen concentrated orange juice – FCOJ) (74). Data from the 2022 Brazilian Horti&Fruti Yearbook indicate that orange juice production in the main Brazilian citrus belt (São Paulo and Triângulo/Southwest Mineiro) was estimated at 820,567 tons in the 2021/22 harvest, equating a 2% reduction compared to the previous period due to climatic problems (3).
Brazil was responsible for three quarters of global exports in the period 2021/22. Brazil exported 1.000 million tons of frozen concentrated orange juice in the period from July 2021 to January 2022, with Europe remaining the main destination. In the same period, there was consumption of 75,000 tons of orange juice in Brazil, and 1.699 million tons worldwide (76).
5 ‘DE RUSSAS’ ORANGE
It is believed that the ‘de Russas’ orange (Figure 1) had already arrived in the Vale do Jaguaribe region through the Portuguese Jesuits in the mid-1930s when they introduced the seeds, and since then were cultivated in the form of unripe plants (propagated by seeds), arousing the interest of farmers, researchers and traders (13).
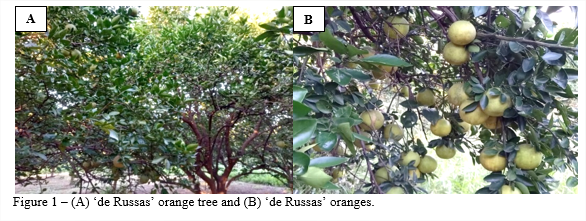
By using the seeds of the ‘de Russas’ orange which had more desirable characteristics, small farmers were able to select mutations that would please the palate of consumers of citrus fruits in the Vale do Jaguaribe region. This orange was the most preferred in the capital of Ceará until the mid-1990s, but is currently unknown by the majority of wholesalers that operate at the Central de Abastecimento of Fortaleza, CE (Ceasa) (11, 10).
5.1 Botanical characteristics
The ‘de Russas’ orange is a variety of the sweet orange (Citrus sinensis (L.) Osbeck). The adult plant is grafted onto ‘Swingle’ citrumelo, its crown is rounded, medium-sized, 3.4 to 4.0 meters high, with a diameter ranging from 2.7 to 3.5 meters at seven years old. Its leaf is large, light green in color and has a curved limb; the fruit has a cylindrical shape, medium to large size, ranging from 190 to 270 g; the rind is smooth or rough, predominantly green and with an orange pulp; the number of seeds per fruit ranges from 2 to 18. The BRS ‘Russas 01’ and BRS ‘Russas 02’ varieties have few seeds (0 to 6), being considered seedless (13, 14).
It has total acidity between 0.6 and 0.9%, total soluble solids from 6.6 to 8.4 ºBrix and the soluble solids/titratable acidity ratio (Ratio) ranging from 7.6 to 15.2. Its productivity ranges from 40 to 60 tons per hectare, with flowering mainly in September and maturation in the mid-season (May and June) (8, 15).
5.2 Propagation
The ‘de Russas’ orange tree plantations were usually planted with non-grafted seedlings (ungrafted plants) without formation pruning which provokes a delay in their development, expanding the size of the plant and making it more vulnerable to diseases, namely rot or gummosis as a major example (11, 14).
Although the Vale do Jaguaribe and the East Coast of Ceará regions have favorable climatic conditions and available water resources, the belt of orange groves in the state is centered on ‘de Russas’ oranges, which are susceptible to Phytophthora gummosis, one of the main Brazilian citriculture diseases (10).
In order to collaborate with the valorization of citrus growing in Ceará, Embrapa Mandioca e Fruticultura in Cruz das Almas (BA) developed the Citriculture Revitalization Project in the Jaguaribe Valley, with support from the Banco do Nordeste and the Federal Institute of Education, Science and Technology of Ceará Campus Limoeiro do Norte, introducing varieties adapted to the region, including the ‘de Russas’ orange, and evaluating different rootstocks under different managements (11).
Grafting is the most used vegetative propagation form (seed-free), which combines a graft and a root holder, joining the two parts to form a new plant with common characteristics. Grafting has been known since the 5th century, but only since the emergence of gomosis on the island of the Azores in 1842 was there a change in citrus cultivation to grafting (77).
The goal of grafting is to gain more productive cultivars, fruits with few seeds and greater juice content, attractive coloration, a balance between sugar and acidity, thereby exerting direct influence on productivity and fruits (78). The first rootstocks used in citrus were sweet oranges which do not tolerate drought and are highly susceptible to gomosis. The most widely used species as a root holder in Brazilian citrus since 1960 is the ‘clove’ (Citrus Limonia Osbeck) due to its tolerance to drought and the citrus tristeza (sadness) virus (79).
5.3 Organic production and family farming
Organic agriculture was idealized by Albert Howard between the years 1925 and 1930 in India, originating as an alternative means of production. It was the opposite of conventional production that was growing rapidly around the world, especially in the United States and Europe, highlighting the great importance of organic matter in the production system. Its main aspect is the non-use of pesticides, chemical fertilizers or synthetic substances which harm the environment. Organic production means the rational use of soil, water, air and other natural resources (80).
The production of organic food and its demand by consumers have shown rapid growth in recent years, especially in Brazil. This increase is in principle associated with rejection of the conventional model, which increasingly uses fertilizers and pesticides which directly affect consumer health; the constant search for new ways to consume these foods; and finally, the advancement of scientific research for innovative tools and resources for use in organic productivity (81, 82).
Despite consumers expressing fear of purchasing a product due to lack of proof that it comes from organic cultivation, several countries have adopted organic certification systems. Institutions accredited by regulatory bodies assess whether the product is in fact organic, granting certificates of conformity and authorization for the use of seals, contributing to identify regulated products (83).
The main Brazilian certification companies are: AAO (Associação de Agricultura Orgânica), ABIO (Associação de Agricultores Biológicos do Rio de Janeiro), Fundação Mokiti Okada, Coolméia, Ecocert, TECPAR (Instituto de Tecnologia do Paraná), IMO Control do Brasil and IBD (Instituto Biodinâmico), with the latter being considered the largest certifier of organic and sustainable products in Latin America. For conformity assessment, these certifiers comply with international procedures and criteria, in addition to the technical requirements established by Brazilian legislation (84).
IBD was accredited by the International Federation of Organic Agriculture Movements (IFOAM) in 1995; it acquired accreditation from the International Organic Accreditation Service and the European market in 1999; it was accredited to the American market in 2002; and, it received MAPA accreditation in 2010 (85).
Law 10,831 in Brazil in 2003 established what an organic production system is and defined its purposes (86). Then, the production and commercialization of organic products was regulated in 2007 by Decree no. 6.323 (87). Normative Instruction no. 18 in 2014 established the single seal of the Brazilian Organic Conformity Assessment System, SisOrg (88). There are also North American, European, Japanese, Swiss, Chinese, and Canadian seals, among others, varying according to the product’s origin certification standards.
In addition to the aforementioned seals, there is Fair for Life, a certification program that reconciles organic and fair trade certification. It was created in 2006 by the Swiss Bio-Foundation in cooperation with the IMO Group and delivered to the Ecocert group in 2014, and is also responsible for all supply chain documentation (89).
There are currently three ways to obtain quality assurance certification for an organic product in Brazil: 1. Hiring an Audit; 2. Connection to a Participatory Guarantee System; and 3. Formation of a Social Control Organization. The seal must appear on certified products according to instructions in the application manual (90).
According to The World of Organic Agriculture statistical yearbook released by the Research Institute of Organic Agriculture (FiBL) and IFOAM in 2019, 3.1 million organic producers were registered worldwide, and the world organic food market reached 106 billion euros, with the United States in the lead, followed by Germany and France (91).
According to the Institute for Applied Economic Research (Instituto de Pesquisa Econômica Aplicada – IPEA), there was an average annual increase of 19% in organic production units between 2010 and 2018, and of almost 17% in the number of organic producers registered in MAPA. Most of these units are concentrated in the Northeast Region (with emphasis on the border between Bahia, Pernambuco, Piauí and Ceará), in the South Region, and in part of the states of São Paulo, Rio de Janeiro and Espírito Santo (92).
Data from the National Register of Organic Producers (Cadastro Nacional de Produtores Orgânicos – CNPO) of the Ministry of Agriculture, Livestock and Supply (MAPA) show that Brazil has more than 23,000 registered organic producers (up until February 3, 2023); significantly responding to the 19,978 registrations in 2019, Ceará specifically has 1,008 registered organic producers, representing an increase of more than 63% compared to 2019, which had only 616 registrations (93).
The aforementioned rates can be justified by the growing search by consumers for organic food, serving as an incentive for family farmers who began to show interest in migrating from conventional to organic production, and those who had already adhered to the new system were driven to increase its productivity and increasing profitability, improving the quality of its products on a sustainable basis and positioning itself more expressively in the organic market. Although the importance of family farming for agricultural development is known, especially in rural areas, there are still obstacles and challenges associated with this model (94).
Some examples of these differences need to be highlighted, such as: obstacles to increasing food production, high deforestation rates in arable areas, insufficiency and inefficiency of agrarian reform, technological differences, instability in technical assistance and rural extension, as well as scarce agricultural financing (95, 96).
For example, the cultivation of ‘de Russas’ oranges in Ceará is basically carried out on small properties with an integration of agriculture and livestock, led by family farming (14). A study carried out demonstrates that although citrus growing in Ceará is a viable path for the socioeconomic stability of these farmers, they do not reach the rural credit line, having a low monthly income of up to three minimum monthly salaries per month (8).
In view of this, it is necessary to disseminate this information to family farmers so that they realize the importance of the agricultural activity they develop for society, as well as for the environment (97).
5.4 Cultivars
The cultivation of the ‘de Russas’ orange has as a historical characteristic the propagation by seeds that were empirically selected in small and medium-sized family farms in the region of the municipality of Russas, which thus led to its name (11).
Ordinance no. 527 of MAPA (98) instituted the National Cultivar Registry (Registro Nacional de Cultivares – RNC), with the aim of organizing, systematizing and controlling the production and sale of seeds and seedlings. The RNC is governed by Law no. 10,711 (99) and regulated by Decree no. 10,586 (100). The eight ‘de Russas’ orange cultivars (BRS ‘Russas 01’ to ‘08’) were registered by Embrapa, with the BRS ‘Russas 03’ cultivar being registered with a date of 2011; and the others in 2013 (101).
Law no. 9,456 (102) instituted the Protection of Cultivars, which is regulated by Decree no. 2,366 of November 5, 1997 (103), within the scope of MAPA, responsible for registrations through the National Service for the Protection of Cultivars (Serviço Nacional de Proteção de Cultivares – SNPC). However, the ‘de Russas’ orange variety has not yet been registered with the SNPC.
CONCLUSION
Based on this review, it appears that the ‘de Russas’ orange currently represents an expressive example of family farming in the interior of the state of Ceará. In addition, that organic cultivation is yet another way of preserving family farming in the semi-arid region of Ceará, reducing hunger and social inequality, contributing to expand citriculture in the State and to the appreciation of the traditional ‘de Russas’ sweet orange variety.
ACKNOWLEDGEMENTS
The authors acknowledge the Instituto Federal de Educação, Ciência e Tecnologia do Ceará (IFCE), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the Master’s scholarship granted to the first author and the Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP scholarship no.: BP4-0172-00061.01.00/2020).
REFERENCES
- Araújo ACC, Budoia SAG. Características físico-químicas e benefícios da farinha de laranja. Nutr Bras. 2019;18(1):49–54.
- Sharma K, Mahato N, Cho MH, Lee YR. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutr. 2017; 34(2):29–46.
- Kist BB, Carvalho C de, Beling RR. Anuário brasileiro de Horti&Fruti 2022. Santa Cruz do Sul: Editora Gazeta Santa Cruz; 2022.
- Guerreiro Neto G, Figueira SRF. Maior dificuldade fitossanitária à produção da laranja no principal cinturão citrícola brasileiro-safras de 2017 a 2019. Citrus R&T. 2021;42(e1066):1–10.
- CitrusBR. Associação Nacional dos Exportadores de Sucos Cítricos. Citricultura gera mais de 38 mil empregos em 2020 [Internet]. 2021 [acesso em 12 Set 2022]. Disponível em: https://citrusbr.com/noticias/citricultura-gera-mais-de-38-mil-empregos-em-2020/
- IBGE. Instituto Brasileiro de Geografia e Estatística. Levantamento Sistemático da Produção Agrícola – LSPA janeiro de 2023 [Internet]. 2023 [acesso em 16 Fev 2023]. Disponível em: https://sidra.ibge.gov.br/home/lspa/ceara
- Almeida CO, Passos OS. Citricultura brasileira em busca de novos rumos: desafios e oportunidades na região Nordeste. Cruz das Almas: Embrapa Mandioca e Fruticultura; 2011.
- Sombra KES, Silva ACC, Loureiro FLC, Uchôa CN. A citricultura como instrumento de preservação da agricultura familiar no semiárido cearense, Brasil. Rev Ext Estud Rurais. 2018a;7(1):21–40.
- IBGE. Instituto Brasileiro de Geografia e Estatística. Produção Agrícola Municipal – PAM 2020 [Internet]. 2021 [acesso em 15 Set 2022]. Disponível em: https://ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/9117-producao-agricola-municipal-culturas-temporarias-e-permanentes.html?=&t=destaques
- Sombra KES, Andrade HM, Loureiro FLC, Oliveira FE, Bastos DC, Uchôa CN. Desenvolvimento de laranjeiras variedade “de Russas” pés francos e clones enxertados no semiárido Cearense. Nativa. 2019;7(1):6–12.
- Passos OS, Souza J da S, Almeida CO de, Carvalho JEB de, Bastos DC, Ledo CA da S. Revitalização da citricultura do Vale Jaguaribe – CE. Cruz das Almas: Embrapa Mandioca e Fruticultura; 2020.
- IBGE. Instituto Brasileiro de Geografia e Estatística. Sidra: Produção Agrícola Municipal – 1990 a 2020 [Internet]. 2020a [acesso em 16 Set 2022]. Disponível em: https://sidra.ibge.gov.br/tabela/5457#resultado
- Passos OS, Soares Filho WS, Barbosa CJ, Cunha Sobrinho AP. Clones da laranjeira ‘DE RUSSAS’. Cruz das Almas: Embrapa Mandioca e Fruticultura; 2013.
- Sombra KES, Silva ACC, Loureiro FLC, Bastos DC. Citricultura desenvolvida na agricultura de base familiar do município de Russas, Ceará. Cult Agron. 2016;25(3):303–316.
- Sombra KES, Silva ACC, Rodrigues AJO, Loureiro FLC, Uchôa CN, Souza PA. Identificação e caracterização físico-química de frutos de laranja de Russas no semiárido cearense, Brasil. Citrus R&T. 2018b;39(e1035):1–9.
- Agustí M. Citricultura. 2 ed. Madrid: Mundi-Prensa Libros; 2003.
- Davies FS, Albrigo D. History, distribution and uses of citrus fruit. In: Donadio LC (Ed.) Citrus. Wallingford: Cab International; 1994.
- Reis B. Incidência de cancro cítrico e produção e qualidade de frutos em laranjeira-de-umbigo ‘Monte Parnaso’ enxertada sobre sete porta-enxertos [dissertação]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2006.
- Silva AS. Determinação de macrocomponentes na laranja (Citrus sinensis): variedades pera e lima, comercializados no município de São Luís – MA [monografia]. São Luiz: Universidade Federal do Maranhão; 2015.
- Dornelles C. Introdução à citricultura. Porto Alegre: Mercado Aberto; 1988.
- Koller OC. Citricultura: laranja, limão e tangerina. Porto Alegre: Editora Rigel; 1994.
- Albrigo LG, Stelinski LL, Timmer LW. History, distribution and uses of citrus fruit. Citrus. 2 ed. Wallingford: Cab International; 2019.
- Moreira CS, Moreira S. História da citricultura no Brasil. In: Rodriguez O, Viegas FCP, Pompeu Júnior J. (Orgs.). Citricultura Brasileira. 2. ed. Campinas: Fundação Cargill; 1991.
- Hasse G. A laranja no Brasil 1500-1987: a história da agroindústria citrícola Brasileira, dos quintais coloniais às fábricas exportadoras de suco do século XX. São Paulo: Duprat & Iobe; 1987.
- Bastos DC, Ferreira EA, Passos OS, Sá JF de, Ataíde EM, Calgaro M. Cultivares copa e porta-enxertos para a citricultura brasileira. Inf Agrop. 2014;35(281):36–45.
- CitrusBR. Associação Nacional dos Exportadores de Sucos Cítricos. A Fruta – Laranja e Suco [Internet]. 2020 [acesso em 06 Set 2022]. Disponível em: http://www.citrusbr.com/laranjaesuco/?ins=20#
- Neves MF, Trombin VG, Milan P, Lopes FF, Cressoni F, Kalaki R. O retrato da citricultura brasileira. Ribeirão Preto: FEA/USP; 2011.
- UFRGS. Universidade Federal do Rio Grande do Sul (org.). Características botânicas [Internet]. 2020 [acesso em 23 Ago 2022]. Disponível em: http://www.ufrgs.br/afeira/materias-primas/frutas/laranja/caracteristicas-botanicas
- Andrade LP de. Extração e biotransformação de flavonoides a partir de resíduo de indústria cítrica [dissertação]. Araraquara: Universidade Estadual Paulista; 2019.
- Cunha Sobrinho AP, Magalhães AFJ, Souza AS, Passos OS, Soares Filho WS. A Cultura dos Citros. Brasília: Embrapa Informação Tecnológica; 2013.
- Oliveira LAE de, Menelau S. Atributos do produto e influências ambientais no comportamento do consumidor de suco de laranja do Distrito Federal. Organ Rurais Agroind. 2017;19(1):60–74.
- TACO. Tabela Brasileira de Composição de Alimentos. 4. ed. Campinas: NEPA-UNICAMP; 2011.
- Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução RDC n° 269, de 22 de setembro de 2005. Aprova o Regulamento Técnico sobre a Ingestão Diária Recomendada (IDR) de proteínas, vitaminas e minerais. Diário Oficial da União. 23 set 2005; Seção 1:372.
- Sdiri S, Cuenca J, Navarro P, Salvador A, Bermejo A. New triploids late-maturing mandarins as a rich source of antioxidant compounds. Eur Food Res Technol. 2020;246(1):225–237.
- Gonçalves D, Lima C, Ferreira P, Costa P, Costa A, Figueiredo W, et al. Orange juice as dietary source of antioxidants for patients with hepatitis C under antiviral therapy. Food Nutr Res. 2017;61(1):1296675.
- Miles EA, Calder PC. Effects of citrus fruit juices and their bioactive components on inflammation and immunity: A narrative review. Front Immunol. 2021;12(2558):1–18.
- Johnson EJ, Russell RM. Beta-carotene. In: Coates P, Blackman M, Cragg GM, Levine MA, Moss J, White JD. (Orgs.) Encyclopedia of Dietary Supplements. New York: Marcel Dekker; 2004.
- Franke AA, Cooney RV, Henning SM, Custer LJ. Bioavailability and antioxidant effects of orange juice components in humans. J Agric Food Chem. 2005; 53(13):5170–5178.
- Buscemi S, Rosafio G, Arcoleo G, Mattina A, Canino B, Montana M, et al. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am J Clin Nutr. 2012;95(5):1089–1095.
- Wang L, Xu H, Yuan F, Fan R, Gao Y. Preparation and physicochemical properties of soluble dietary fiber from orange peel assisted by steam explosion and dilute acid soaking. Food Chem. 2015;185(10):90–98.
- Silva JCS, Orlando EA, Rebellato AP, Pallone J. A Optimization and validation of a simple method for mineral potential evaluation in citrus residue. Food Anal Methods. 2017;10(6):1899–1908.
- Brasil. Atos do Poder Executivo. Decreto nº 6.871, de 04 de junho de 2009. Regulamenta a Lei nº 8.918, de 14 de julho de 1994, que dispõe sobre a padronização, a classificação, o registro, a inspeção, a produção e a fiscalização de bebidas. Diário Oficial da União. 05 jun 2009; Seção 1:20.
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento/Secretaria de Defesa Agropecuária. Instrução Normativa n° 37, de 01 de outubro de 2018. Estabelece os parâmetros analíticos de suco e de polpa de frutas e a listagem das frutas e demais quesitos complementares aos padrões de identidade e qualidade já fixados. Diário Oficial da União. 08 out 2018; Seção 1:23.
- Lemos LMC, Siqueira DL, Salomão LCC, Cecon PR, Lemos JP. Características físico-químicas da laranja-Pera em função da posição na copa. Rev Bras Frutic. 2012;34(4):1091–1097.
- Coelho BES, Duarte VM, Silva LFM, Sousa KSM, Figueiredo Neto A. Atributos físico-químicos de frutos de laranja ‘Pêra’ produzidos sob sistemas de cultivo orgânico e convencional. Rev Bras Meio Ambiente. 2019;5(1):128–137.
- Amaro KC, Tadini CC. The optimal time-temperature conditions for orange juice microwave-assisted pasteurization. LWT. 2021;150:111907.
- Chitarra MIF, Chitarra AB. Pós-colheita de frutos e hortaliças: fisiologia e manuseio. Lavras: ESAL/FAEP; 2005.
- Fellows PJ. Tecnologia do processamento de alimentos: Princípios e práticas. 4. ed. Porto Alegre: Artmed; 2019.
- Costa J de O. Determinação do teor de vitamina C em polpas de frutas congeladas por Iodimetria: uma opção para o controle de qualidade? [trabalho de conclusão de curso]. Vitória de Santo Antão: Universidade Federal de Pernambuco; 2016.
- Cunha KD, Silva PRD, Costa ALF, Fonseca S da, Teodoro AJ, Koblitz MGB. Estabilidade de ácido ascórbico em sucos de frutas frescos sob diferentes formas de armazenamento. Braz J Food Technol. 2014;17(2):139–145.
- Cardoso JA da C, Rossales RR, Limons B, Reis SF, Schumacher B de O, Helbig E. Teor e estabilidade de vitamina C em sucos in natura e industrializados. Mundo Saúde. 2015;39(4):460–469.
- Fonseca NC, Petean PG da C. Determinação dos parâmetros cinéticos de degradação da vitamina C em suco de laranja. Rev Bras Iniciaç Cient. 2018;5(3)46–59.
- Saqueti BHF, Alves ES, Ponhozi IBS, Castilho PA, Castro MC, Souza PM, et al. Viabilidade da obtenção de polpa de acerola (Malpighia spp) microencapsulada e liofilizada: Uma revisão. Res Soc Dev. 2021;10(2):e30410212536.
- Romeo R, Bruno AD, Piscopo A, Medina E, Ramírez E, Brenes M, et al. Effects of phenolic enrichment on vitamin C and antioxidant activity of commercial orange juice. Brazilian J Food Technol. 2020;23(e2019130):1–12.
- Souza LF da S, Domingos LF, Farias VL da S, Luzia DMM. Avaliação físico-química e estabilidade do ácido ascórbico em sucos de frutas comercializados no município de Frutal, Minas Gerais. Revista Verde. 2017;12(4):791–797.
- Dallago RM, Pascuetti Tres B, Denti AF, Oro CED, Dornelles Venquiaruto L. Avaliação de sucos de laranja artesanais produzidos na Microrregião de Erechim. Persp. 2020;44(167):15–24.
- Rosa JS da, Godoy RL de O, Oiano Neto J, Campos R da S, Matta VM da, Freire CA, et al. Desenvolvimento de um método de análise de vitamina C em alimentos por cromatografia líquida de alta eficiência e exclusão iônica. Food Sci Technol. 2007;27(4):837–846.
- Fabricio DS. Determinação de vitamina C em suco de frutas in natura e industrializados por cromatografia líquida e titulação iodométrica [trabalho de conclusão de curso]. Londrina: Universidade Tecnológica Federal do Paraná; 2018.
- Mendonça JKA, Fontana TC. Variação da concentração de vitamina C em sucos de laranja armazenados com diferentes condições de luminosidade e temperatura. Rev Thema. 2021;19(1):95–106.
- Fan G, Lu W, Yao X, Zhang Y, Wang K, Pan S. Effect of fermentation on free and bound volatile compounds of orange juice. Flavour Fragr J. 2009;24(5):219–225.
- Marques S. Otimização das condições de extração por micro-extração em fase sólida (SPME) de compostos voláteis de suco de tangerina [monografia]. São Carlos: Universidade de São Paulo; 2016.
- Marques S. Influência de diferentes porta-enxertos na composição química dos óleos essenciais e do suco de tangerina Fremont IAC 543 [dissertação]. São Carlos: Universidade de São Paulo; 2019.
- Mariano APX, Ramos ALCC, Oliveira Júnior AH de, García YM, Paula ACCFF de, Silva MR, et al. Optimization of Extraction Conditions and Characterization of Volatile Organic Compounds of Eugenia klotzschiana O. Berg Fruit Pulp. Molecules. 2022;27(3):935.
- Schenkel D, Lemfack MC, Piechulla B, Splivallo R. A meta-analysis approach for assessing the diversity and specificity of belowground root and microbial volatiles. Front Plant Sci. 2015;6(707):1–11.
- García YM, Rufini J, Campos MP, Guedes MN, Augusti R, Melo JO. SPME fiber evaluation for volatile organic compounds extraction from acerola. J Braz Chem Soc. 2019;30(2):247–255.
- Pinheiro CL, Taranto OP, Tomaz E. Study of volatile organic compounds (VOCs) emitted by orange bagasse drying process. Process Saf Environ Prot. 2018;114(2):16–24.
- Bezerra RV, Oliveira HMBF de, Lima CMBL, Diniz M de FFM, Pêssoa H de LF, Oliveira Filho AA de. Atividade antimicrobiana dos monoterpenos (R)-(+)-citronelal,(S)-(-)-citronelal e 7-hidroxicitronelal contra cepa de Bacillus Subtilis. Rev Uningá. 2019;56(2):62–69.
- Felipe LO, Bicas JL. Terpenos, aromas e a química dos compostos naturais. Quim Nova. 2017;39(2):120–130.
- Canuto KM, Garruti D dos S, Magalhães HCR. Microextração em fase sólida: métodos analíticos práticos para extração de compostos voláteis de frutas. Embrapa Agroindústria Tropical-Comunicado Técnico (INFOTECA-E); 2011.
- Nascimento RF do, Lima ACA de, Barbosa PGA, Silva VPA da. Cromatografia gasosa: aspectos teóricos e práticos. Fortaleza: Imprensa Universitária; 2018.
- Figueiredo IB, Tocchini RP, Bordoni LCAG. Observações sobre os componentes voláteis e alterações organolépticas em sucos concentrados de laranja pera. Bol Inst Technol Alim. 1979;16(2):191–207.
- Brealey O. Aroma e compostos voláteis da laranja [dissertação]. Campinas: Universidade Estadual de Campinas; 1972.
- Mirhosseini H, Salmah Y, Nazimah SAH, Tan CP. Solid-phase microextration for headspace analysis of key volatile organic compounds in orange beverage emulsion. Food Chem. 2007;105(4):1659–1670.
- FAO. Food and Agriculture of the United Nations. Statistical Databases [Internet]. 2022 [acesso em 2023 Fev 16]. Disponível em: http://www.fao.org/faostat/en/#rankings/commodities_by_country
- IBGE. Instituto Brasileiro de Geografia e Estatística. Produção Agrícola Municipal – PAM. Rio de Janeiro: IBGE; 2020b.
- USDA. United States Department of Agriculture. Citrus: World Markets and Trade. Washington: Foreign Agricultural Service; 2022.
- Buzaglo GB. Combinações copa – porta-enxerto alternativas para produção de laranja doce na região metropolitana de Manaus-AM [dissertação]. Manaus: Universidade Federal do Amazonas; 2021.
- Crasque J, Cerri Neto B, Souza GAR de, Costa RJ, Arantes L de O, Arantes SD, et al. Características físico-químicas de frutos de laranja em diferentes porta-enxertos. Int J Dev Res. 2020;10(08):39534–39539.
- Costa DP, Stuchi ES, Girardi EA, Moreira AS, Gesteira A da S, Coelho Filho MA, et al. Less is more: A hard way to get potential dwarfing hybrid rootstocks for Valencia sweet orange. Agriculture. 2021;11(4):354–374.
- Sousa MJD de, Cajú MAD, Oliveira CPA. A importância da produção agrícola orgânica na agricultura familiar. Id on Line Rev Psic. 2016;10(31):82–100.
- Lourenço AV, Schneider S, Gazolla M. A agricultura orgânica no Brasil: um perfil a partir do censo agropecuário 2006. Exten Rur. 2017;24(1):42–61.
- Maas L, Malvestiti R, Vergara LGL, Gontijo LA. Agricultura orgânica: uma tendência saudável para o produtor. Cadernos de C&T. 2018;35(1):75–92.
- Martinelli JT. Sistema de processamento orgânico em indústrias produtoras de alimentos: um estudo de caso [trabalho de conclusão de curso]. Florianópolis: Universidade Federal de Santa Catarina; 2019.
- Moraes MD de, Oliveira NAM de. Produção orgânica e agricultura familiar: obstáculos e oportunidades. Rev Desenvolv Socioecon Debate. 2017;3(1):19–37.
- IBD. Instituto Biodinâmico. Certificações [Internet]. [acesso em 10 Set 2022]. Disponível em: https://www.ibd.com.br/certificacoes/
- Brasil. Atos do Poder Legislativo. Lei nº 10.831, de 23 de dezembro de 2003. Dispõe sobre a agricultura orgânica e dá outras providências. Diário Oficial da União. 24 dez 2003a; Seção 1:8.
- Brasil. Atos do Poder Executivo. Decreto nº 6.323, de 27 de dezembro de 2007. Regulamenta a Lei nº 10.831, de 23 de dezembro de 2003, que dispõe sobre a agricultura orgânica, e dá outras providências. Diário Oficial da União. 28 dez 2007; Seção 1:2.
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Instrução Normativa nº 18, de 20 de junho de 2014. Institui o selo único oficial do Sistema Brasileiro de Avaliação da Conformidade Orgânica e estabelece os requisitos para a sua utilização. Diário Oficial da União. 23 jun 2014; Seção 1:2.
- Kemper L, Partzsch L. Saving water while doing business: Corporate agenda-setting and water sustainability. Water. 2019;11(297):1–30.
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Regularização da Produção Orgânica [Internet]. 2021 [acesso em 15 Set 2022]. Disponível em: https://www.gov.br/agricultura/pt-br/assuntos/sustentabilidade/organicos/regularizacao-da-producao-organica
- FiBL. Research Institute of Organic Agriculture. IFOAM. Organics International. The World of Organic Agriculture. Statistics & Emerging Trends [Internet]. 2021 [acesso em 12 Set 2022]. Disponível em: https://www.fibl.org/fileadmin/documents/shop/1150-organic-world-2021.pdf
- IPEA. Instituto de Pesquisa Econômica Aplicada. Produção e consumo de produtos orgânicos no mundo e no Brasil. Texto para discussão. Brasília: Ipea; 2020.
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Orgânicos: Cadastro Nacional de Produtores Orgânicos [Internet]. 2022 [acesso em 22 Fev 2023]. Disponível em: https://www.gov.br/agricultura/pt-br/assuntos/sustentabilidade/organicos/cadastro-nacional-produtores-organicos
- Duarte LC, Weber C, Amorim G dos S, Spanevello RM, Lago A. Mercados para a agricultura familiar. Braz J Dev. 2020;6(7):44370–44384.
- Paula MM de, Kamimura QP, Silva JLG da. Mercados institucionais na agricultura familiar: dificuldades e desafios. Rev Polit Agric. 2014;23(1):33–43.
- Pinho GA de, Pedroso OS, Sá Durlo R de, Guedes SNR. A agricultura orgânica como nicho de atividades para a agricultura familiar no Brasil: dificuldades e possibilidades. Rev Iniciativa Econ. 2015;2(1):1–23.
- Sousa WD, Melo FKE de, Sousa EP de. Sustentabilidade da agricultura familiar no município de Barro – CE. Rev Gest Sustent Ambiental. 2017;6(2):302–327.
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Portaria n° 527, de 30 de dezembro de 1997. Institui o Registro Nacional de Cultivares – RNC, e procedimentos de inscrição. Diário Oficial da União. 07 jan 1998; Seção 1:100.
- Brasil. Atos do Poder Executivo. Lei n° 10.711, de 05 de agosto de 2003. Dispõe sobre o Sistema Nacional de Sementes e Mudas e dá outras providências. Diário Oficial da União. 06 ago 2003b; Seção 1:1.
- Brasil. Ato do Poder Executivo. Decreto nº 10.586, de 18 de dezembro de 2020. Regulamenta a Lei nº 9.456, de 25 de abril de 1997. Institui a Proteção de Cultivares, dispõe sobre o Serviço Nacional de Proteção de Cultivares – SNPC, e dá outras providências. Diário Oficial da União. 21 dez 2020; Seção 1:2.
- SRNC. Sistema de Registro Nacional de Cultivares. CGSM. Coordenação-Geral de Sementes e Mudas. DSV. Departamento de Sanidade Vegetal e Insumos Agrícolas. DAS. Secretaria de Defesa Agropecuária. MAPA. Ministério da Agricultura, Pecuária e Abastecimento. Registro Nacional de Cultivares – RNC [Internet]. 2021 [acesso em 04 Set 2022]. Disponível em: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/sementes-e-mudas/registro-nacional-de-cultivares-2013-rnc-1
- Brasil. Atos do Poder Legislativo. Lei n° 9.456, de 25 de abril de 1997. Institui a Lei de Proteção de Cultivares e dá outras providências. Diário Oficial da União. 28 abr 1997a; Seção 1:8241.
- Brasil. Atos do Poder Executivo. Decreto nº 2.366, de 05 de novembro de 1997. Lei nº 10.711, de 5 de agosto de 2003. Dispõe sobre o Sistema Nacional de Sementes e Mudas. Diário Oficial da União. 06 nov 1997b; Seção 1:25333.